Take a Journey of Discovery with RXTE - Classroom Activity

The Electromagnetic Spectrum
Although it would seem that the human eye gives us a pretty accurate view of the world, we are literally blind to much of what surrounds us. A whole Universe of color exists, only a thin band of which our eyes are able to detect; an example of this visible range of color is the familiar concept of a rainbow or a spectrum. The optical spectrum ranges in color from reds and oranges up through blues and purples. Each of these colors actually corresponds to a different energy of light. The colors or energies of light that our eyes cannot see also have names that are familiar to us.
| We listen to radios,
|
 |
 |
we eat food heated in microwaves,
|
we have X-rays taken of our broken bones.
|
 |
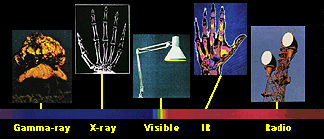
Yet many times we do not realize that radio, X-ray, and microwave are really different energies of light! The entire range of energies of light, including both light we can see and light we cannot see, is called the electromagnetic spectrum. It includes, from highest energy to lowest: gamma-rays, X-rays, ultraviolet, optical, infrared, microwaves, and radio waves.
Because we can only see visible light, we are put at a disadvantage when we study the Universe, because the Universe is actively emitting light at all these different energies.
Energy? Wavelength? Frequency?
Light has different colors because it has different energies. This is true whether we are talking about red and blue visible light, or infrared (IR) and X-ray light. Of all the colors in the visible spectrum, red light is the least energetic and blue is the most. Beyond the red end of the visible part of the spectrum lie infrared and radio light, both of which have lower energy than visible light. Above the blue end of the visible spectrum lies the higher energies of ultraviolet light, X-rays, and finally, gamma-rays.
Because of light's unique properties, it can be described not only in terms of its energy, but also its wavelength, or its frequency. X-rays and gamma-rays are usually described in terms of energy, optical and infrared light in terms of wavelength, and radio in terms of frequency. This is a scientific convention that allows the use of the units that are the most convenient for describing whatever energy of light you are looking at.
Wavelength is the distance between two peaks of a wave, usually measured in meters (m). Frequency is the number of cycles of a wave to pass some point in a second. The units of frequency are thus cycles per second, or Hertz (Hz). Energy in astronomy is often measured in electron Volts, or eV. In X-ray astronomy, we use kilo-electron Volts or keV. "Kilo" means 1000, so a keV is 1000 electron Volts.
Since light can act like both a particle and a wave, we say that light has particle-wave duality. We call particles of light photons. Low-energy photons (i.e. radio) tend to behave more like waves, while higher energy photons (i.e. X-rays) behave more like particles. This is an important difference because it affects the way we build instruments to measure light.


